Animals Medical Devices Commentary
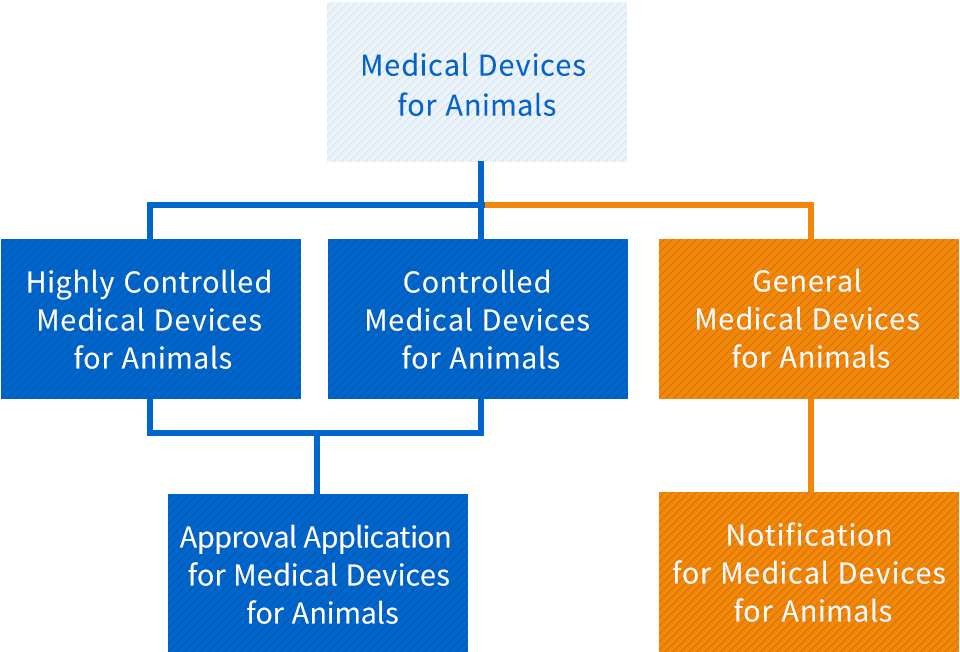
Highly Controlled Medical Devices for Animals
As there is a risk of a serious impact on the life and health of animals in the event of side effects or impairment of function, the appropriate management is required, and the Minister of Agriculture, Forestry and Fisheries designates specially controlled medical devices for animals by listening to the opinion of Pharmaceutical Affairs and Food Sanitation Council (Article 2, Paragraph 5 of the Act)
- Example
-
- Closed-circuit anesthesia system
- Artificial heart valve
- Artificial heart-lung machines
- Hemodialysis apparatus
- Pacemakers
- Closed circulation infant incubators
Controlled Medical Devices for Animals
As there is a risk of an impact on the life and health of animals in the event of adverse effects or functional disorders other than specially controlled medical devices, and the Minister of Agriculture, Forestry and Fisheries designates specially controlled medical devices for animals by listening to the opinion of Pharmaceutical Affairs and Food Sanitation Council (Article 2, Paragraph 6 of the Act)
- Example
-
- Anesthesia apparatus and anesthesia respiratory sacs and gas absorbers
- Breathing aid
- Artificial internal organs
- Incubator
- X-ray generator and X-ray tube device
- Lithotrogical instruments
- Clinical chemistry analyzer
- Bone setting supply
General Medical Devices for Animals
As there is a risk of an impact on the life and health of animals in the event of side effects or impairment of function other than Specially controlled medical devices and Management medical devices, the appropriate management is required, and the Minister of Agriculture, Forestry and Fisheries designates specially controlled medical devices for animals by listening to the opinion of Pharmaceutical Affairs and Food Sanitation Council (Article 2, Paragraph 7 of the Act)
- Example
-
- Medical disinfectants
- Medical sterilization water device
- Stethoscope
- Thermometer
- Urine chemistry analyzers
- Rigid endoscopes
- Medical tweezers
- Single-use needles for injections