FEATURE
RAPID provides support for foreign or domestic market entry in Japan, with expertise in navigating Japan’s challenging regulatory environment.
It is also possible to get regulatory application approval for regenerative medical products. We will help you to get over that process smoothly and quickly as your marketing authorization license holder (DMAH).
Our specialized team will offer consultation with quick response and secure pricing.
Please feel free to contact us for consultation and quotation.

-
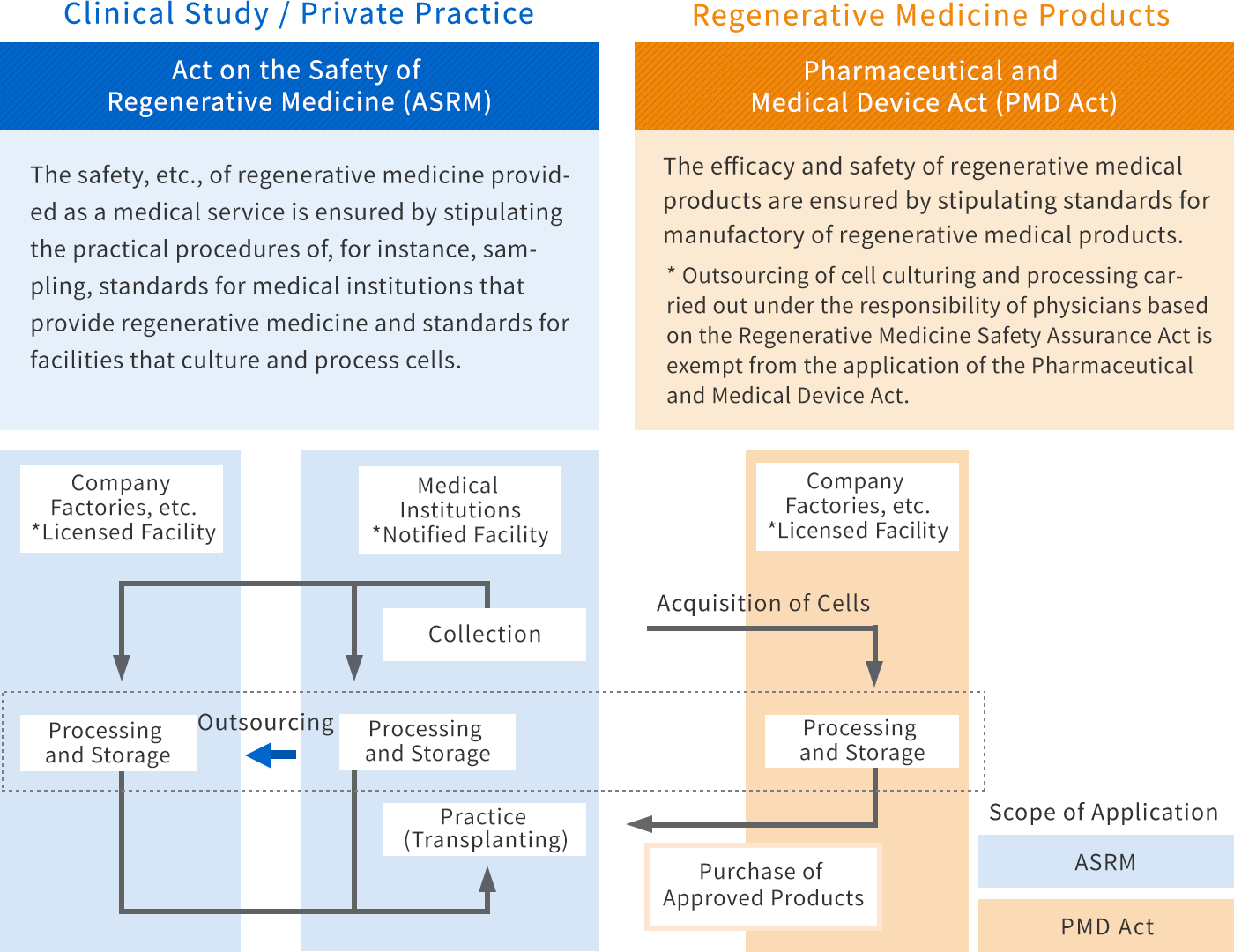
Regulatory application for regenerative medical related products and imports your products in Japan
For more information, please visit Medical Device Development Services
-

-
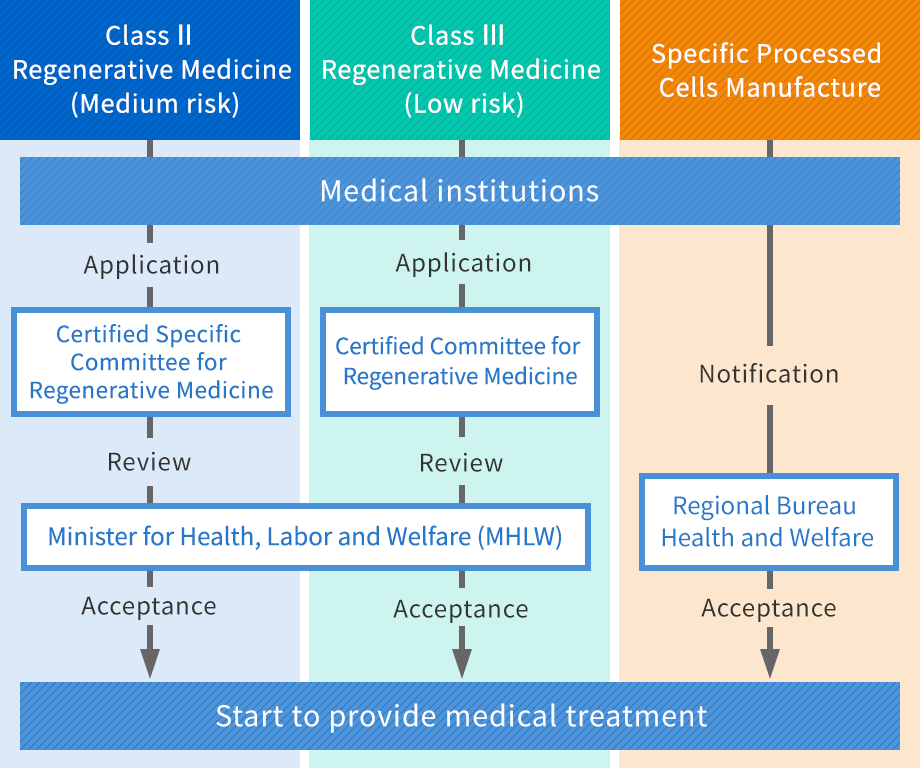
Preparation of provision plan of Class Ⅱ / Class Ⅲ regenerative medicines (It will take 1~2 months)
Preparation of partial application / Notification of Specific Processed Cells Manufacture
150,000 yen〜
Making all application
500,000 yen〜

-
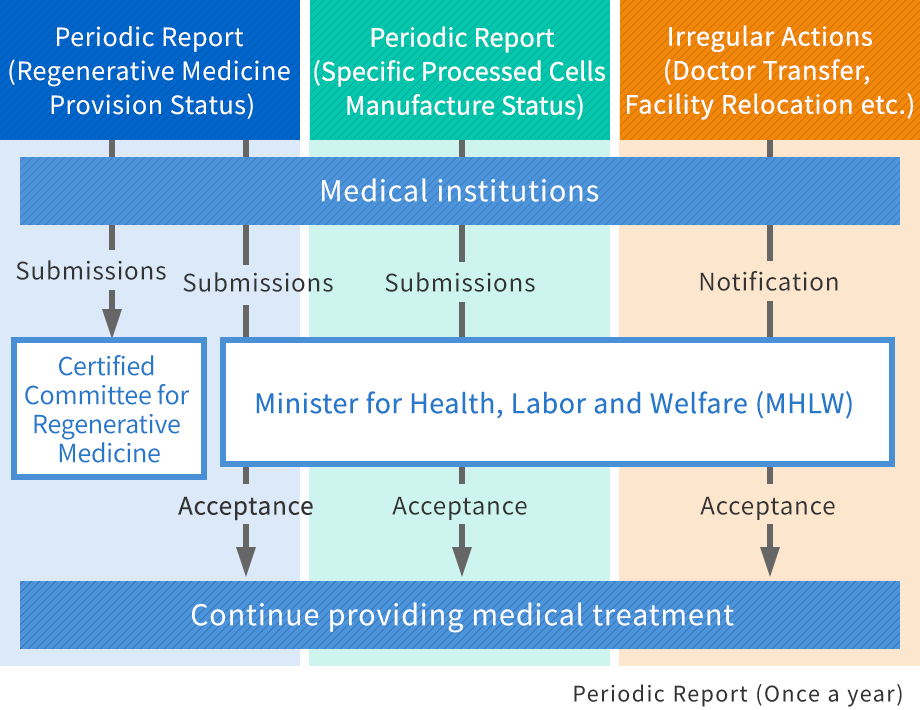
Support for periodic reports after approval
100,000 yen〜