-
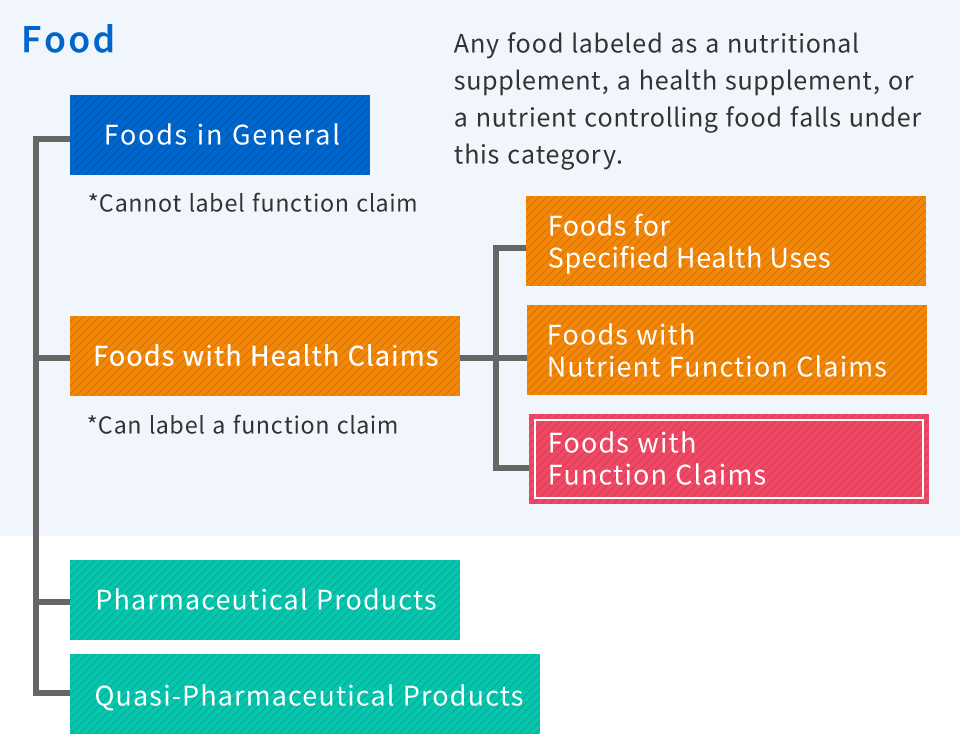
What are Foods with Function Claims?
Foods with function claims are functional labeling systems for foods that was introduced in April 2015 and are different from food for specified health uses (FOSHU) and functional nutritional food. To sell foods with function claims, at the responsibility of operator, it is necessary to label the functionality on the food according to scientific basis and notify the Commissioner of the Consumer Affairs Agency before selling.
-
FOODS WITH
FUNCTION CLAIMS SERVICES
The new system of Foods with Function Claims has been introduced in 2015 in Japan. This is different from Foods for Specified Health Uses and Foods with Nutrient Function Claims. RAPID provides regulatory support for companies want to enter a new field of supplements Japan market in compliance with the food labeling standards and PMD Act (former Japanese Pharmaceutical Law).
To be labeled as Foods with Functional Claims
would be significantly improve sales in Japan.
RAPID will assist reducing your development costs and improve sales.
- We provide support for making submission documents with reasonable price.
- You can make a label with the specified health effects can be achieved (i.e., helpful for maintaining and promoting health) such as “Helps maintain good GI condition” or “Slows fat absorption.”
- We can offer consultation with professional knowledge when you do not have enough time for research and development.
- If you want to differentiate products from competitors, it is possible to be labeled with a function claim which is based on scientific evidence.
- Consumers can make a product choice without misunderstanding the information.
RAPID provides services for consultation on industry lowest price.
Our specialized team have the expertise to support for the food labeling standards and
PMD Act (former Japanese Pharmaceutical Law) in compliance with the law.Please feel free to contact us for consultation and quotation.
Procedures Required for
Marketing Foods with Function Claims
-
- 01Initial Consultation
- First of all, RAPID will hear your request.
- Determining whether your product is subject to Foods with Function Claims.
- Consultation on what health claims (effects and efficacy) to be labeled.
-
- 02Preliminary Research
- Providing the results of the preliminary research.
- Explanation of the period and cost until obtaining Foods with Function Claims.
- Explanation what tests are required.
-
- 03Product Safety
- Product safety must be evaluated and explained by one of the following methods.
- Evaluating the history of consumption by humans based on actual intake data.
- Collecting secondary information through databases.
- Identify any interactions of functional substances with drugs.
-
- 04Quality Control System
- A system for ensuring safety in terms of sanitation and quality control during the production and manufacturing processes must be established and described.
- The location and management structure of the production plant.
- Analytical methods for quantitative tests on functional-involved components and components requiring security of safety.
-
- 05Collecting Adverse Health Events
- For prevention of the occurrence and spread of adverse health outcomes, a system to collect and report information must be established.
-
- 06Substantiating the Product Effectiveness *
- The scientific evidence for the proposed function claims must be explained by one of the following methods.
- Systematic literature reviews on a finished product or functional substances.
- Clinical trials with a finished product (equivalent level of study required for Foods for Specified Health Uses).
-
- 07Labeling the Product Properly
- Product labelling must be in accordance with the Food Labelling Standards.
- Development indication that conforms to the scientific basis and is passed on to consumers.
-
- 08Submission
- Sixty days prior to the targeted launch date, a completed notification and related documents must be submitted to the Secretary-General of the Consumer Affairs Agency.
- Basic information is submitted to the Secretary-General of the Consumer Affairs Agency and receiving the ID.
- Each Foods with Function Claims shall be submitted by using the ID.
-
- 09Product Sales
- Once accepted by the Secretary-General of the Consumer Affairs Agency, A notification number will be given, and the product can be marketed as Foods with Function Claims.
Evaluation of the Claimed Effect *
There are two ways to provide scientific evidence when evaluating a claimed effect.
- Systematic Literature Reviews
Performing a literature search by following certain rules and evaluating the data comprehensively. -
-
01Retrieving and Filtering Literature
The authors of a systematic literature review retrieve research papers by searching databases that contain articles on such as clinical trials and observational studies* related to the claimed effect of the finished product or the functional substances in the finished product according to a pre-defined methodological approach prepared by the authors. It is not acceptable to intentionally retrieve only studies reporting favorable results in relation to the claimed effect. *Studies that involve human subjects and evaluate the effects of consumption of a certain substance or food on health outcomes.
-

-
-
-
02Scientifically Evaluating the Claimed Effect
All retrieved studies are filtered by assessing against inclusion/exclusion criteria such as characteristics of finished product, target population, a proposed claim, etc. Those studies that meet the pre-determined criteria are then grouped according to whether or not their results support the effectiveness of the finished product or the functional substances.
-

-
-
-
03Disclosing the Evaluation Process and Results
The totality of evidence on the claimed effect of finished product or functional substances should be considered by taking all favorable, unfavorable, and equivocal results together. To permit reproducibility of the systematic literature review, all of the processes of the systematic literature review, such as used databases, set of keywords, the inclusion/exclusion criteria, and the title of excluded studies, need to be thoroughly documented and submitted.
-

-
- Clinical Trials
- Clinical trials are conducted in humans to examine the functionality, safety, and other aspects of the final product. This is performed based on a clinical trial protocol prepared using statistical techniques to confirm the effect of the displayed functionality, and at the same time, the safety of the product can be confirmed. It will be an important test for safer and more appropriate use.
The contents of trials will be considered scientifically and ethically by the Ethics Review Board established to protect the rights of those participating in trials and the trial will be started after it has been approved.
In this way, it is not only time-consuming to carry out the trial, and it is also costly to carry out the trial for humans. On the other hand, it works very effectively as an evidence to prove the functionality and safety of the product. It will be possible to respond in clinical trials when systematic reviews are difficult in areas where less literature has been published or research has not progressed.
CONTACT
For more information about RAPID services and inquiries, please feel free contact us.
Phone +81-50-5361-6640 9:00-17:00 Japan Standard Time
